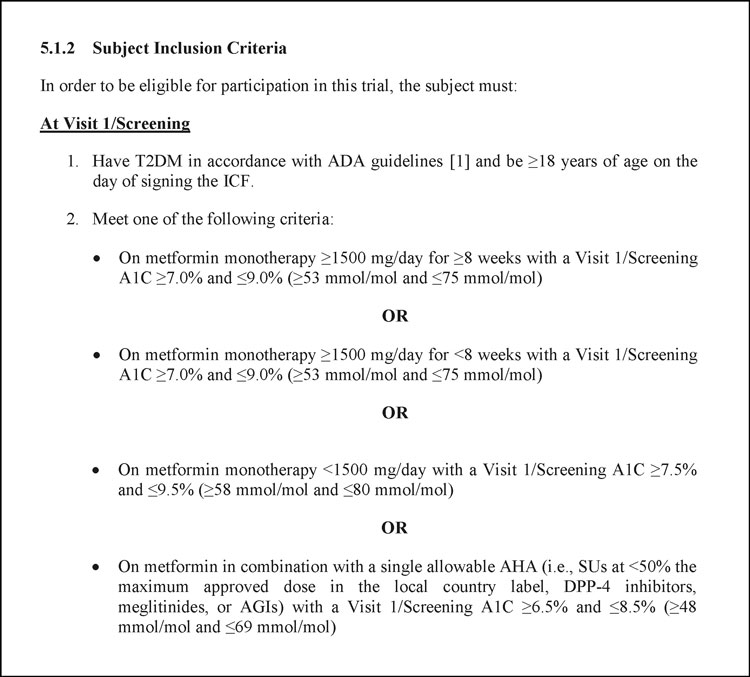
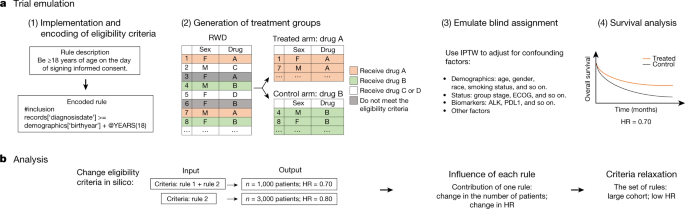
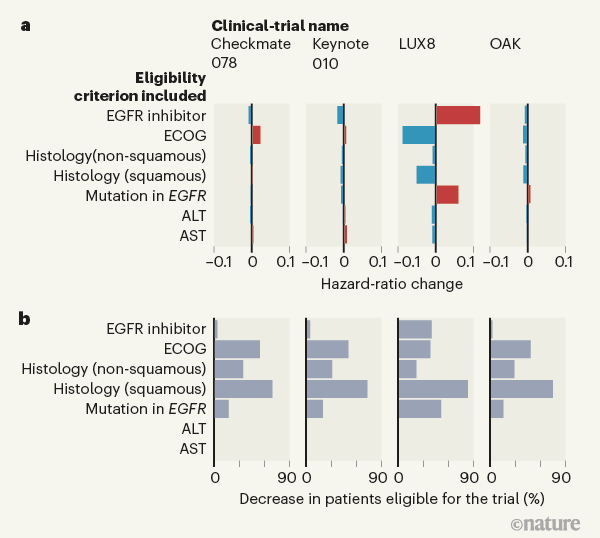
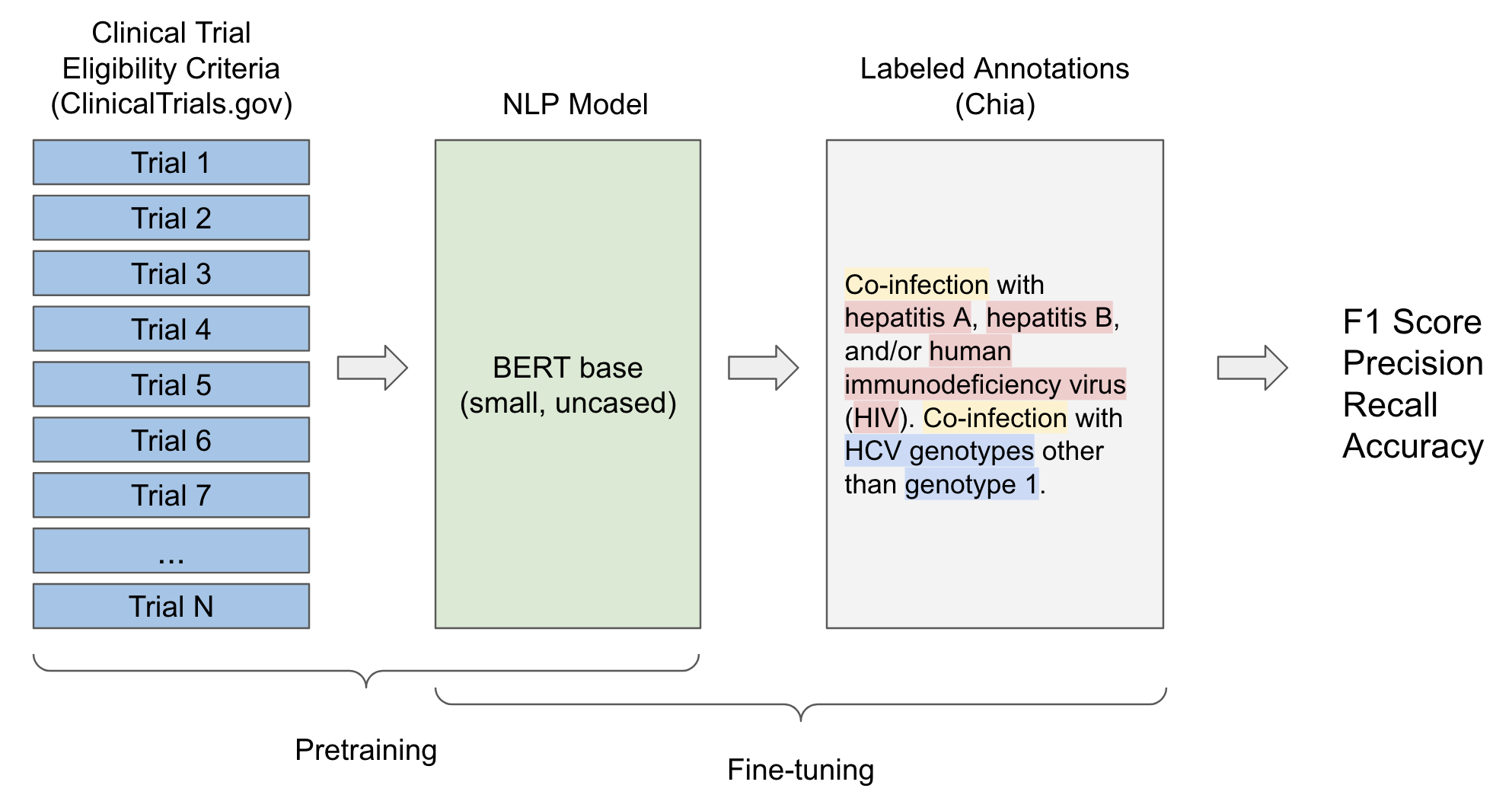
![PDF] Comparison of Eligibility Criteria Between Protocols, Registries, and Publications of Cancer Clinical Trials. | Semantic Scholar PDF] Comparison of Eligibility Criteria Between Protocols, Registries, and Publications of Cancer Clinical Trials. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/62bcc7885dac29517026f3ba577045d3b93dbb7b/2-Table2-1.png)
PDF] Comparison of Eligibility Criteria Between Protocols, Registries, and Publications of Cancer Clinical Trials. | Semantic Scholar
Evaluating the frequency of English language requirements in clinical trial eligibility criteria: A systematic analysis using ClinicalTrials.gov | PLOS Medicine

Transforming Clinical Trial Eligibility Criteria to Reflect Practical Clinical Application | American Society of Clinical Oncology Educational Book

Barriers to Enrollment in Non-small Cell Lung Cancer Therapeutic Clinical Trials - Journal of Thoracic Oncology
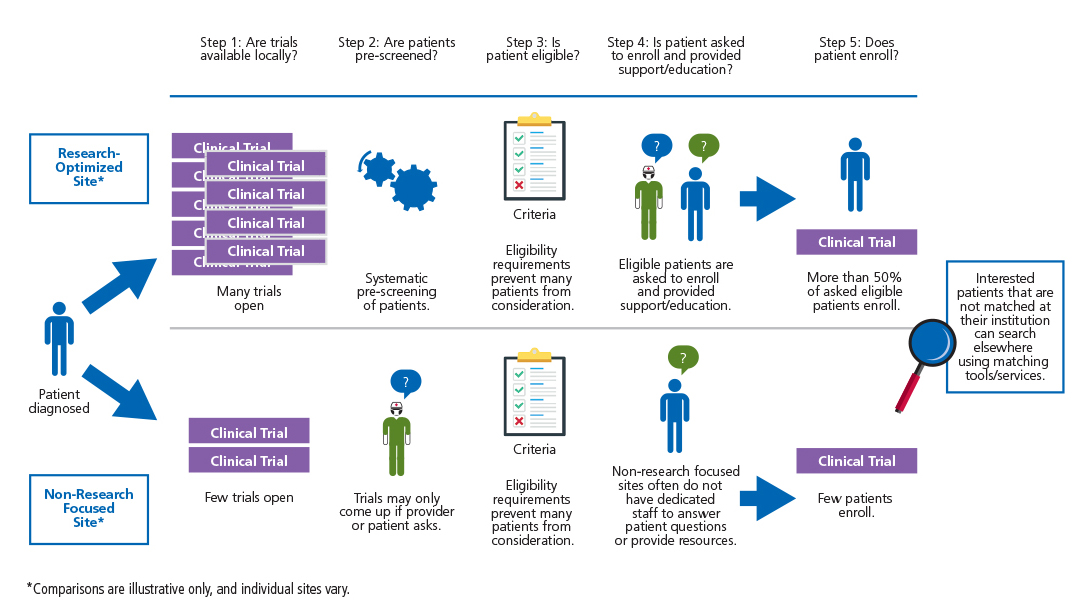
Barriers to Patient Enrollment in Therapeutic Clinical Trials for Cancer | American Cancer Society Cancer Action Network

A systematic review describes models for recruitment prediction at the design stage of a clinical trial - Journal of Clinical Epidemiology

Re-Evaluating Eligibility Criteria for Oncology Clinical Trials: Analysis of Investigational New Drug Applications in 2015 | Journal of Clinical Oncology
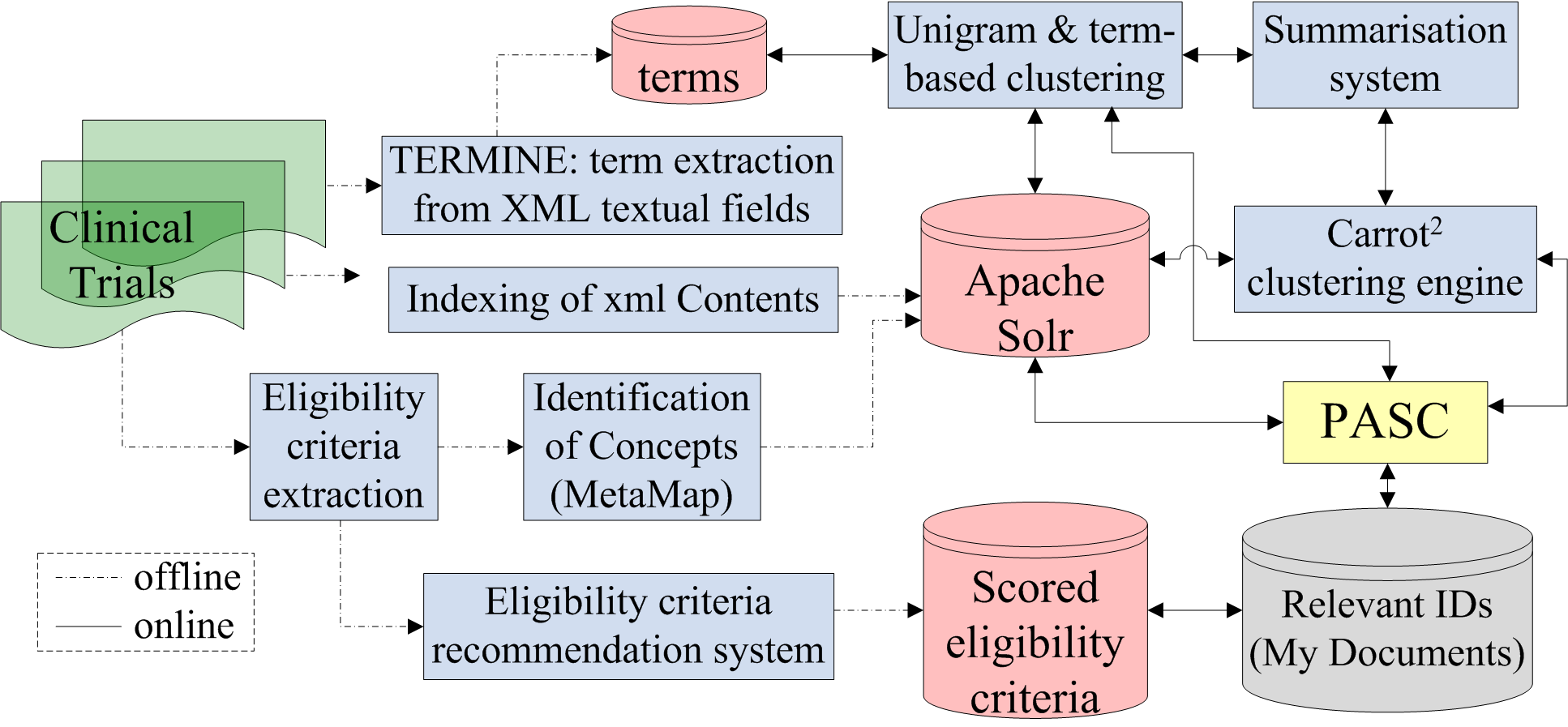
![PDF] Automated classification of eligibility criteria in clinical trials to facilitate patient-trial matching for specific patient populations | Semantic Scholar PDF] Automated classification of eligibility criteria in clinical trials to facilitate patient-trial matching for specific patient populations | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/259f4fca7905dbb313f32a7bcd717bd08785cbd1/4-Figure1-1.png)
PDF] Automated classification of eligibility criteria in clinical trials to facilitate patient-trial matching for specific patient populations | Semantic Scholar







![PDF] Structuring Clinical Trial Eligibility Criteria with the Common Data Model | Semantic Scholar PDF] Structuring Clinical Trial Eligibility Criteria with the Common Data Model | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/5e868e8e7f9a5d6c706ac180b5e59aa0ce45f35f/2-Table1-1.png)