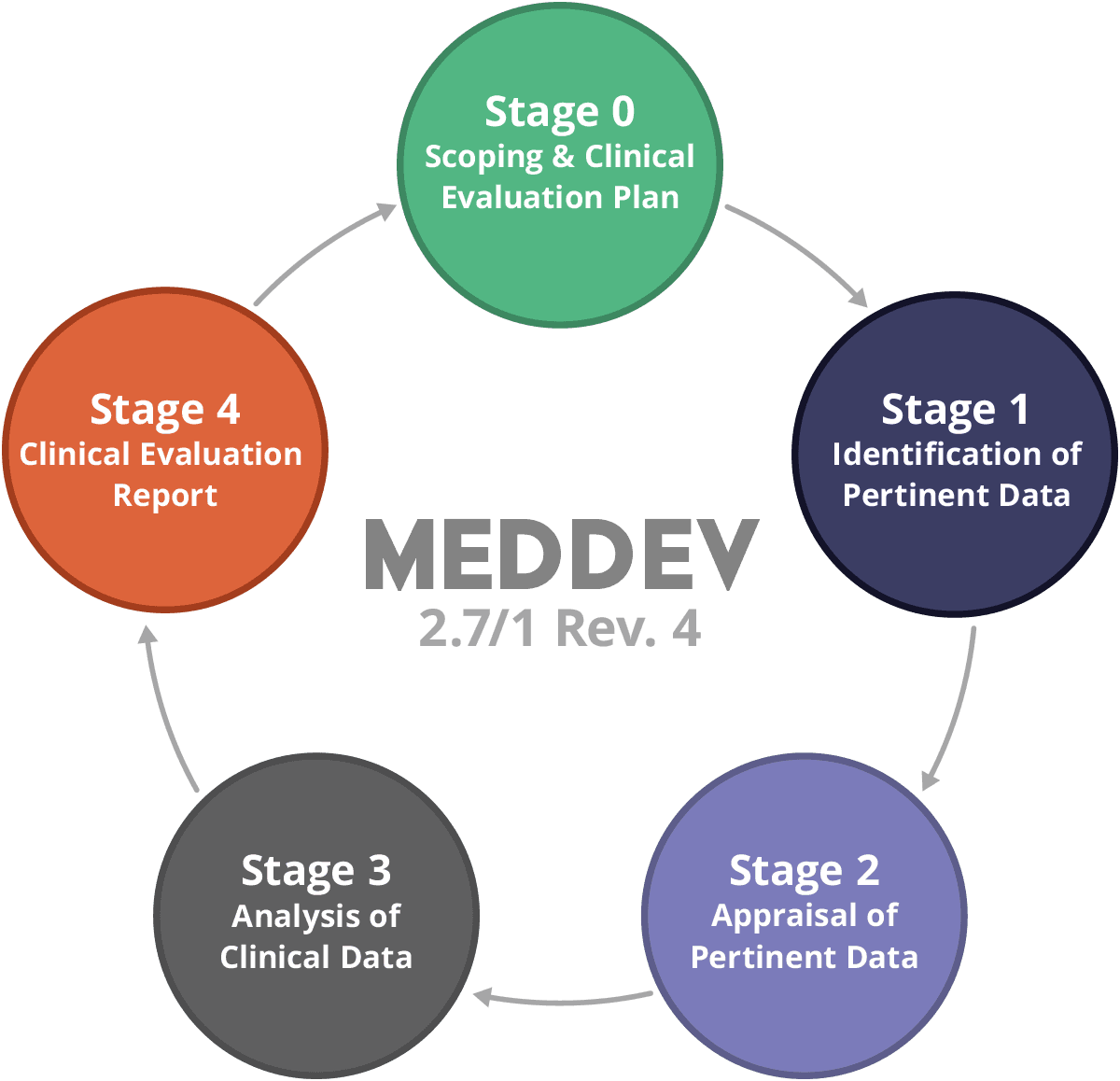
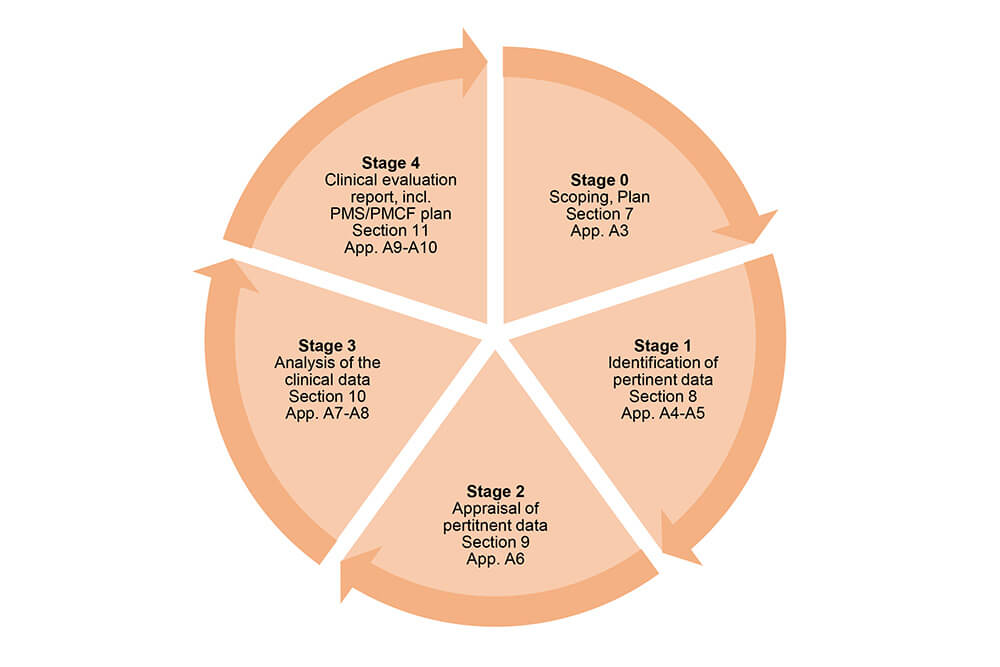
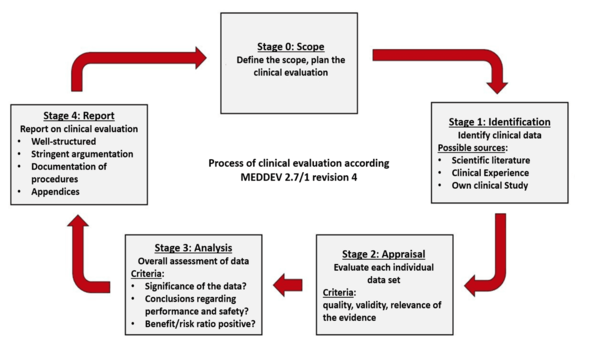
MEDDEV 2.7.1 Rev 4: Implementing New Requirements for Clinical Evaluation Reports (CER) Demo - YouTube

How to perform a clinical evaluation of medical devices – Part 3 – Suggested Table of Contents for the Clinical Evaluation Report – CER – Medical Device Expert News

Clinical evaluation: is your Clinical Evaluation Report (CER) compliant with MEDDEV 2.7/1 rev. 4? - Thema Med

MEDDEV 2.7/1 rev 4: How will your clinical evaluation change? - Medical Device Academy Medical Device Academy